Herlev Pharma provides expert clinical trial services for pharma- and biotech companies. We ensure safe, compliant handling of APIs, intermediates, and finished products - supporting your clinical studies at every stage, from storage and import to transport and regulatory compliance.
Herlev Pharma bridges the gap between CMC, development activities, and GMP by providing comprehensive, regulatorily compliant services that cover the entire value chain — from development to production and delivery. In this way, we ensure your clinical studies and product development always meet the highest quality and safety standards.
How We Connect CMC, Development Activities, and GMP
At Herlev Pharma, we understand the importance of creating a seamless link between CMC (Chemistry, Manufacturing, and Controls), development activities, and GMP (Good Manufacturing Practice). Our services are specifically designed to support and integrate these key elements throughout pharmaceutical production and clinical trials.
Integrating CMC and Development Activities
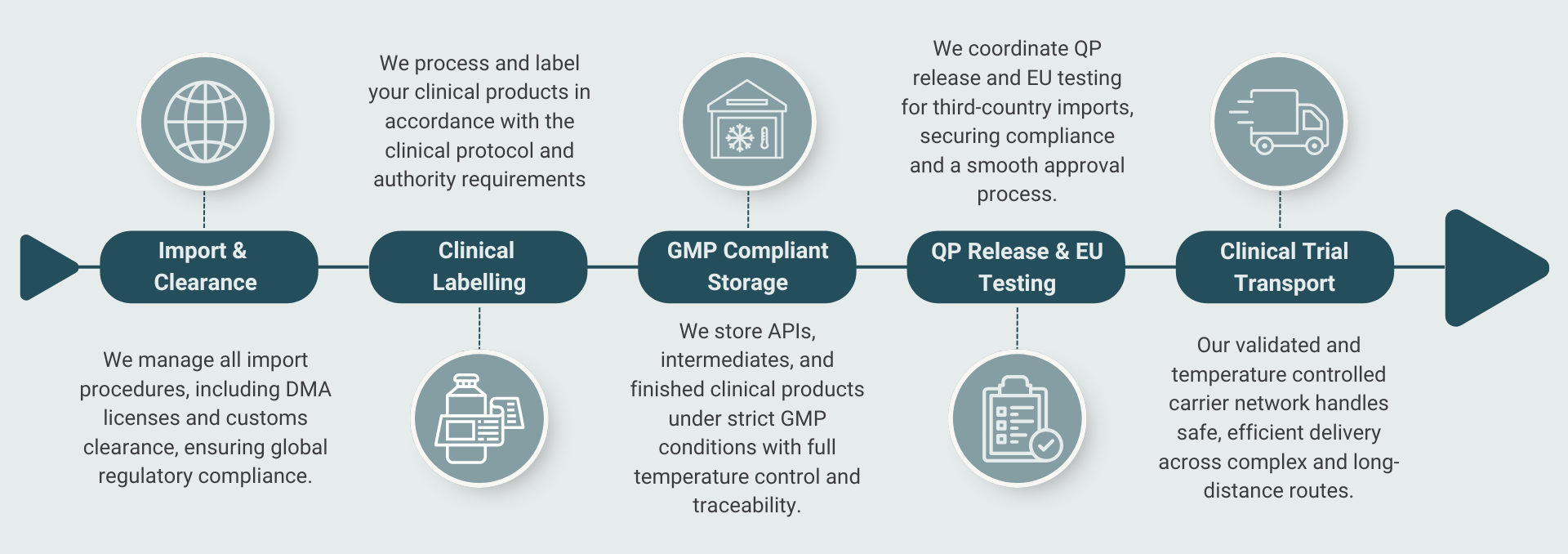
We help ensure that the technical and regulatory requirements established during the development phase (CMC) are consistently followed throughout production and clinical studies. This applies especially to the storage, import, transport, and documentation of clinical products. Our expertise allows you to focus on product development while we handle all practical and regulatory aspects.
Supporting GMP at Every Stage
GMP is at the core of our approach. We ensure that the storage, handling, and transport of your products are performed in accordance with the highest quality standards. This includes:
Flexible and Validated Transport Solutions
We continuously qualify and onboard new carriers, ensuring we always have 2-3 validated solutions available. This enables us to select the best option for your specific needs, project timelines, and budget — while guaranteeing that GMP requirements are met at every step, including temperature-controlled cargo.
QP Release and EU Testing
For products from third countries, we assist with the entire QP release and EU testing process, ensuring your products meet all regulatory requirements before use in clinical studies.
Contact us today to learn how we can support your clinical studies, handling, and logistics needs — ensuring your pharmaceutical products are always in safe and capable hands.